Phasing Out Animal Research: Are US Labs Ready for AI Alternatives?
As the U.S. considers reducing animal testing, are labs ready to adopt AI and organ-on-a-chip alternatives? A deep dive into the science, policy, and global trends shaping the future of research.
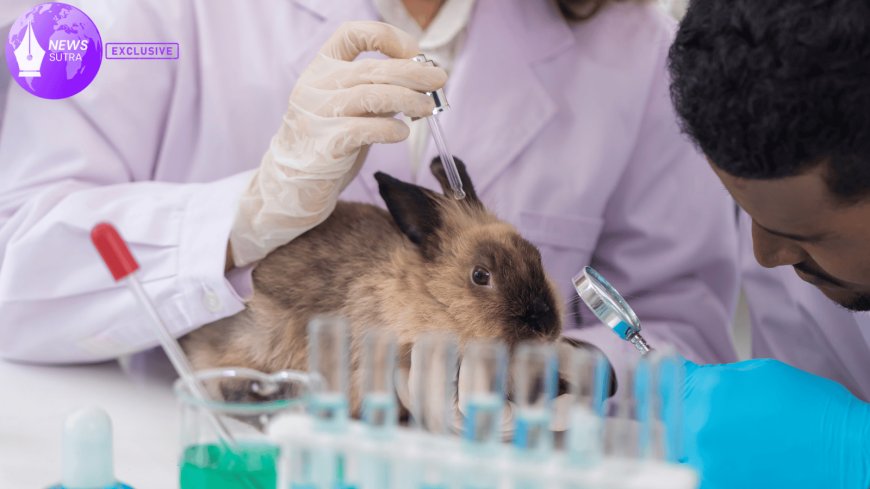
For decades, animal testing has been the backbone of biomedical research in the United States, from drug development to toxicology studies. But with growing ethical concerns and scientific advances in artificial intelligence (AI) and biotechnology, policymakers, scientists, and the public are asking whether the country is prepared to move beyond animal models. The transition is no longer a far-off ideal — it is a question pressing down on U.S. labs today.
The Push to End Animal Testing
The momentum against animal research has been steadily growing. In 2022, the U.S. Congress passed legislation encouraging federal agencies to explore alternatives to animal use in drug testing. The Food and Drug Administration (FDA) has since begun reviewing non-animal models, signaling a major shift in regulatory frameworks.
Advocacy groups point to moral concerns, citing millions of animals used annually in testing, often under painful conditions. Yet, the issue is not only ethical — it’s scientific. Critics argue that animal models frequently fail to predict human outcomes. A study published in the British Medical Journal estimated that more than 90% of new drugs that pass animal testing later fail in human trials.
The Rise of Organ-on-a-Chip and AI Models
The most promising alternatives today come from a combination of biotechnology and machine learning. One notable development is organ-on-a-chip technology, which uses human-derived cells on microchips to mimic organ functions. These chips allow scientists to study drug reactions in ways far more relevant to humans than mice or rats.
At the same time, AI-driven models are transforming toxicology and pharmacology research. By training algorithms on large datasets of past experiments, researchers can predict how compounds might interact with human biology without ever involving a living creature.
The National Institutes of Health (NIH) has invested heavily in these areas, with pilot projects already demonstrating that machine learning systems can accurately predict carcinogenic risks. If scaled, these models could significantly reduce the need for animal trials.
For readers interested in the technical details, the NIH’s National Center for Advancing Translational Sciences offers a comprehensive overview of its current organ-on-a-chip initiatives.
Challenges Facing U.S. Labs
Despite the promise, there are steep challenges. First, infrastructure in most labs remains geared toward traditional animal models. Transitioning to AI-based or chip-based platforms requires new equipment, retraining of personnel, and large upfront investments.
Second, regulatory acceptance is still limited. While agencies like the FDA are beginning to consider alternatives, full-scale approval of new therapies based solely on non-animal methods is rare. This creates hesitation in the private sector, where companies fear investing in tools regulators may not yet recognize.
Lastly, there are questions about accessibility. Smaller labs, particularly those in universities without strong funding, may struggle to adopt high-cost, cutting-edge alternatives. This raises concerns about whether the scientific community as a whole — not just elite institutions — can benefit from the shift.
Lessons from Abroad
The U.S. is not alone in grappling with this issue. The European Union has already banned animal testing for cosmetics and is advancing non-animal safety assessments for chemicals. Similarly, countries like Japan and South Korea are investing in AI toxicology platforms to reduce reliance on animal studies.
Comparing these global trends highlights the U.S.’s mixed position: a leader in innovation but slower to reform its regulations compared to Europe. For a comprehensive international perspective, Humane Society International provides detailed reports on alternative testing methods worldwide.
What Comes Next?
Experts believe the path forward will be gradual. Instead of an overnight ban, the U.S. is likely to adopt a hybrid model, where animal studies are supplemented — and eventually replaced — by AI and chip-based systems as evidence of reliability grows.
Policy reforms will play a crucial role. If agencies accelerate validation standards for alternative technologies, more labs will embrace the shift. If not, the U.S. risks lagging behind in the next era of biomedical research.
Conclusion
The conversation about phasing out animal research is not simply about ethics — it is about the future of science. With AI and organ-on-a-chip innovations advancing rapidly, the United States faces a pivotal choice: continue to rely on outdated models or fully invest in technologies that could make research more humane, efficient, and accurate.
For now, U.S. labs stand at a crossroads. Whether they seize this moment depends as much on political will and regulatory flexibility as on scientific innovation itself.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0








































































